Cluster Analysis in R
14 minute read
Introduction
- What is Clustering?
- Clustering is the classification of data objects into similarity groups (clusters) according to a defined distance measure.
- It is used in many fields, such as machine learning, data mining, pattern recognition, image analysis, genomics, systems biology, etc.
- Machine learning typically regards data clustering as a form of unsupervised learning.
- Why Clustering and Data Mining in R?}
- Efficient data structures and functions for clustering
- Reproducible and programmable
- Comprehensive set of clustering and machine learning libraries
- Integration with many other data analysis tools
- Useful Links
Data Preprocessing
Data Transformations
Choice depends on data set!
- Center and standardize
- Center: subtract from each value the mean of the corresponding vector
- Standardize: devide by standard deviation
- Result: Mean = 0 and STDEV = 1
- Center and scale with the
scale()function- Center: subtract from each value the mean of the corresponding vector
- Scale: divide centered vector by their root mean square (rms): [ x_{rms} = \sqrt[]{\frac{1}{n-1}\sum_{i=1}^{n}{x_{i}{^2}}} ]
- Result: Mean = 0 and STDEV = 1
- Log transformation
- Rank transformation: replace measured values by ranks
- No transformation
Distance Methods
List of most common ones!
- Euclidean distance for two profiles X and Y:
[ d(X,Y) = \sqrt[]{ \sum_{i=1}^{n}{(x_{i}-y_{i})^2} }]
- Disadvantages: not scale invariant, not for negative correlations
- Maximum, Manhattan, Canberra, binary, Minowski, …
- Correlation-based distance: 1-r
- Pearson correlation coefficient (PCC):
$$r = \frac{n\sum_{i=1}^{n}{x_{i}y_{i}} - \sum_{i=1}^{n}{x_{i}} \sum_{i=1}^{n}{y_{i}}}{ \sqrt[]{(\sum_{i=1}^{n}{x_{i}^2} - (\sum_{i=1}^{n}{x_{i})^2}) (\sum_{i=1}^{n}{y_{i}^2} - (\sum_{i=1}^{n}{y_{i})^2})} }$$- Disadvantage: outlier sensitive
- Spearman correlation coefficient (SCC)
- Same calculation as PCC but with ranked values!
- Pearson correlation coefficient (PCC):
There are many more distance measures
- If the distances among items are quantifiable, then clustering is possible.
- Choose the most accurate and meaningful distance measure for a given field of application.
- If uncertain then choose several distance measures and compare the results.
Cluster Linkage
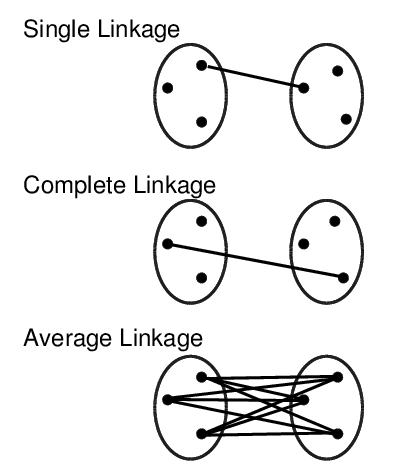
Clustering Algorithms
Hierarchical Clustering
Overview of algorithm
- Identify clusters (items) with closest distance
- Join them to new clusters
- Compute distance between clusters (items)
- Return to step 1
Hierarchical clustering: agglomerative Approach
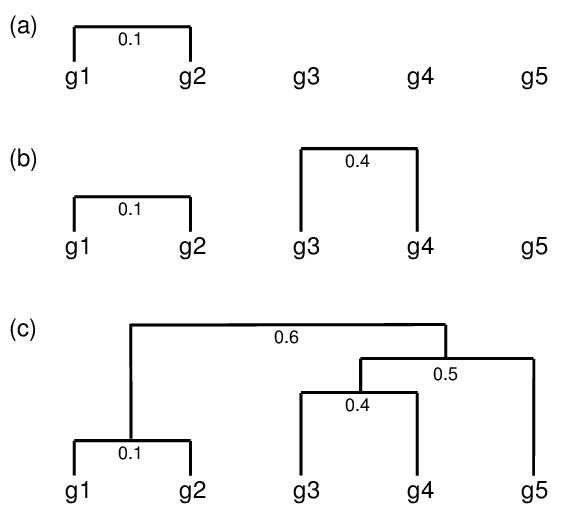
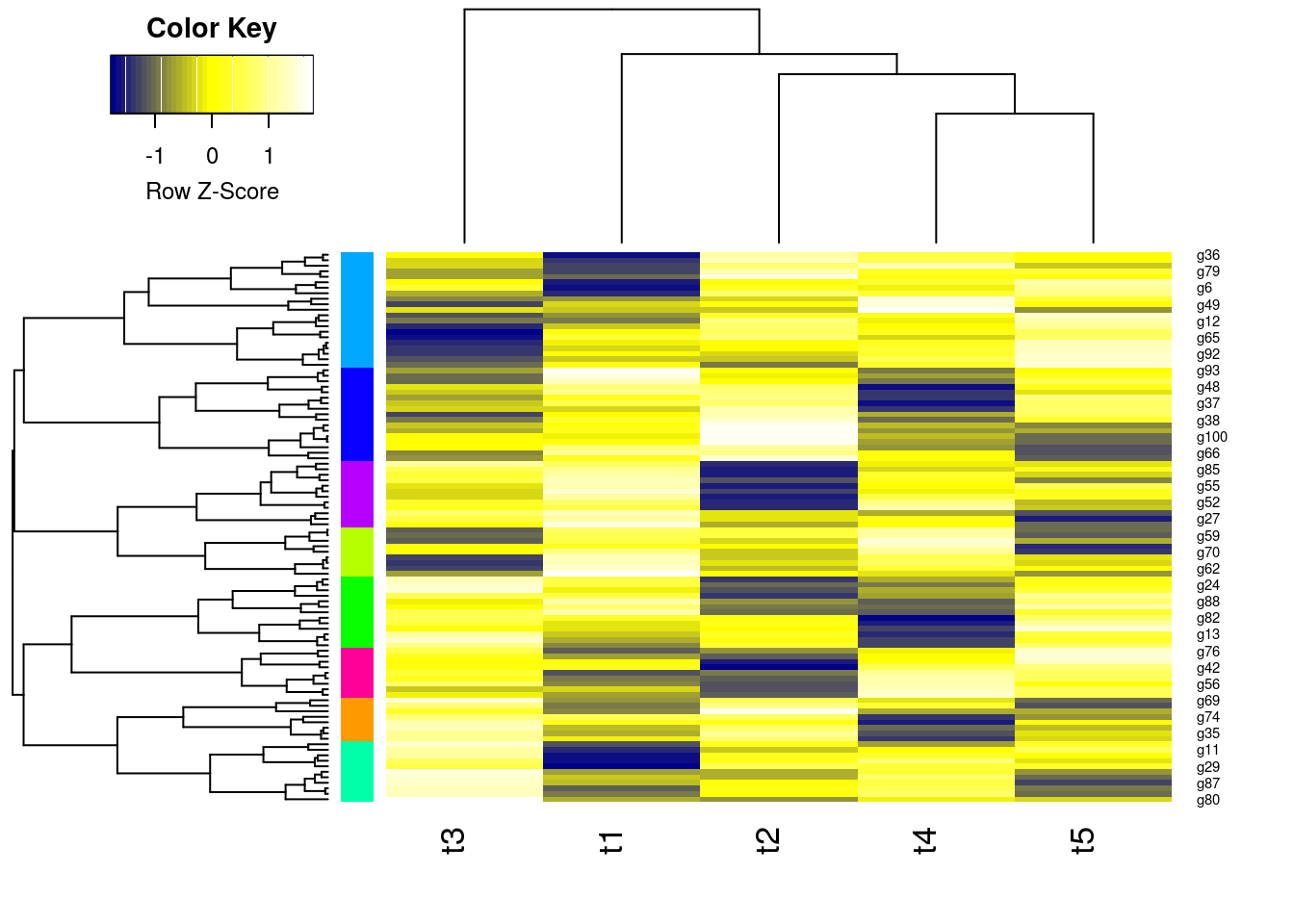
Hierarchical Clustering with Heatmap
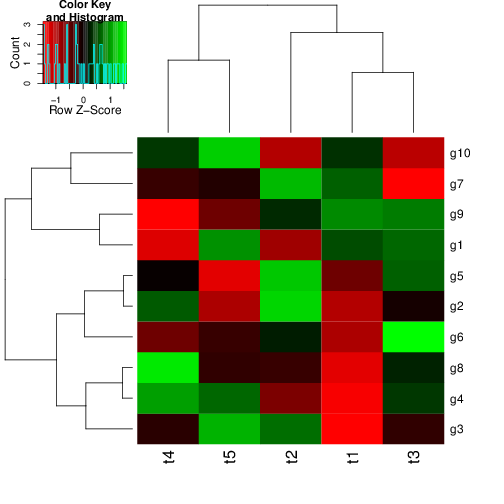
- A heatmap is a color coded table. To visually identify patterns, the rows and columns of a heatmap are often sorted by hierarchical clustering trees.
- In case of gene expression data, the row tree usually represents the genes, the column tree the treatments and the colors in the heat table represent the intensities or ratios of the underlying gene expression data set.
Hierarchical Clustering Approaches
- Agglomerative approach (bottom-up)
- R functions:
hclust()andagnes()
- R functions:
- Divisive approach (top-down)
- R function:
diana()
- R function:
Tree Cutting to Obtain Discrete Clusters
- Node height in tree
- Number of clusters
- Search tree nodes by distance cutoff
Examples
Required packages
The code sections in this tutorial make use of the following R/Bioc packages: c("ggplot2", "gplots", "pheatmap", "RColorBrewer", "ape", "ComplexHeatmap", "scatterplot3d"). If they are not available on a system, then they need to be installed with BiocManager::install() first.
Using hclust and heatmap.2
Note, with large data sets consider using flashClust which is a fast implementation of hierarchical clustering.
library(gplots)
y <- matrix(rnorm(500), 100, 5, dimnames=list(paste("g", 1:100, sep=""), paste("t", 1:5, sep="")))
heatmap.2(y) # Shortcut to final result
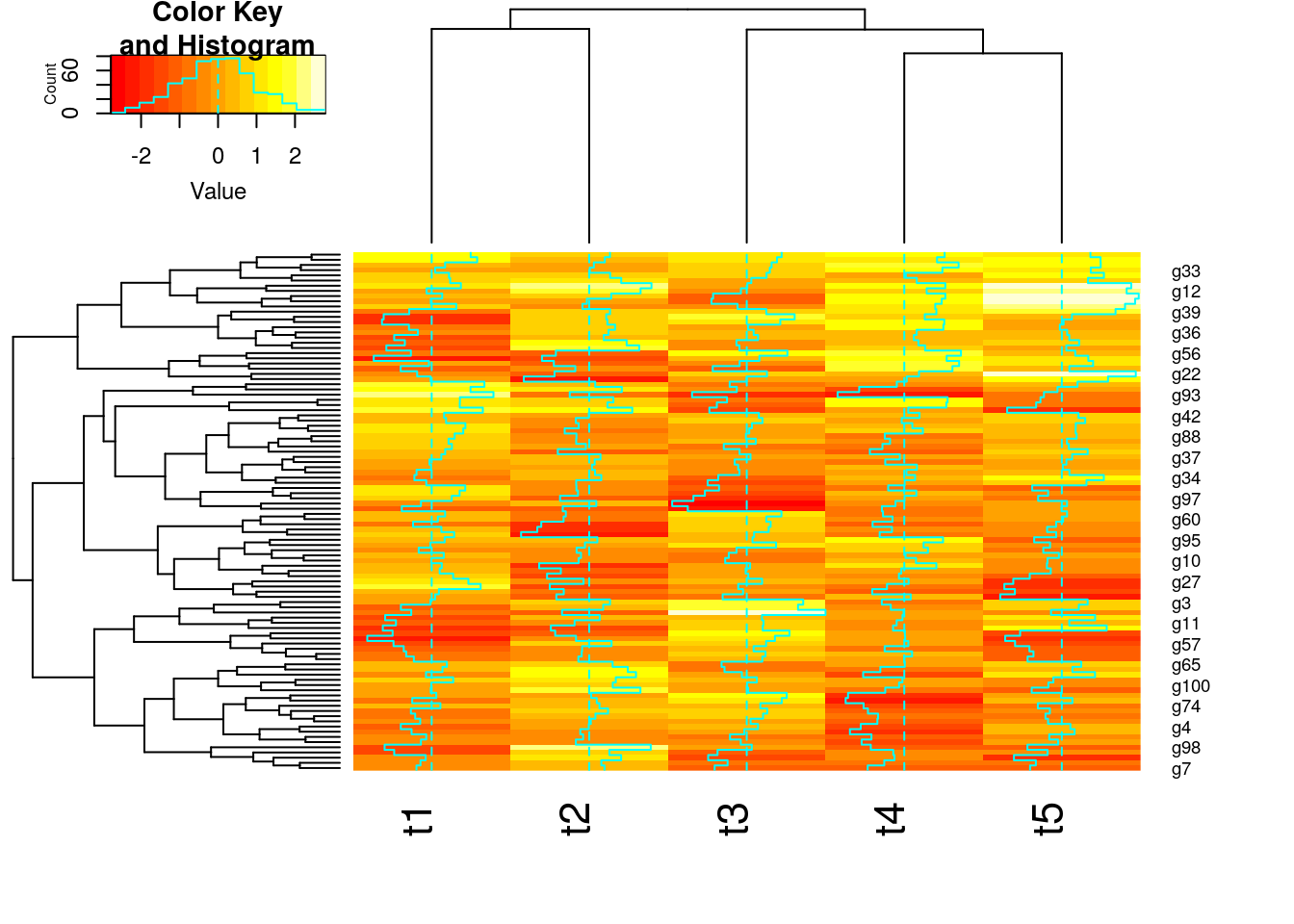
Stepwise Approach with Tree Cutting
## Row- and column-wise clustering
hr <- hclust(as.dist(1-cor(t(y), method="pearson")), method="complete")
hc <- hclust(as.dist(1-cor(y, method="spearman")), method="complete")
## Tree cutting
mycl <- cutree(hr, h=max(hr$height)/1.5); mycolhc <- rainbow(length(unique(mycl)), start=0.1, end=0.9); mycolhc <- mycolhc[as.vector(mycl)]
## Plot heatmap
mycol <- colorpanel(40, "darkblue", "yellow", "white") # or try redgreen(75)
heatmap.2(y, Rowv=as.dendrogram(hr), Colv=as.dendrogram(hc), col=mycol, scale="row", density.info="none", trace="none", RowSideColors=mycolhc)
K-Means Clustering
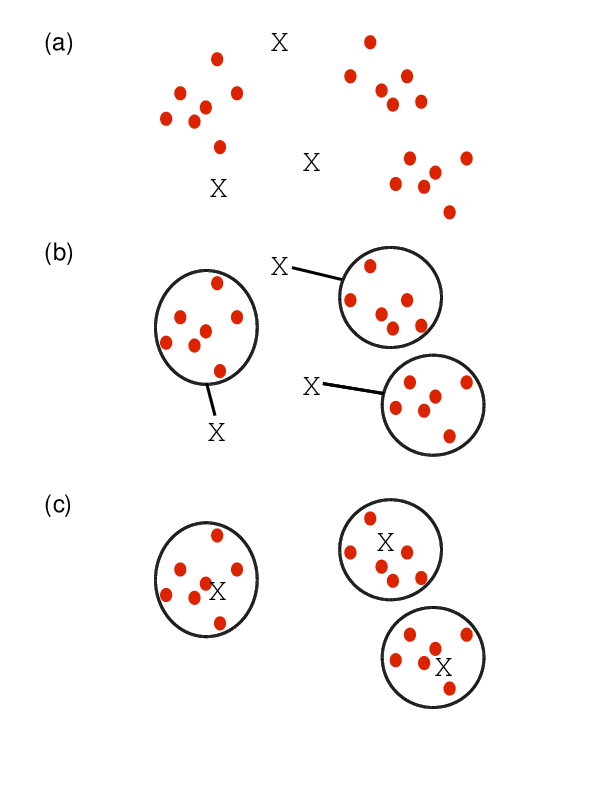
Overview of algorithm
- Choose the number of k clusters
- Randomly assign items to the k clusters
- Calculate new centroid for each of the k clusters
- Calculate the distance of all items to the k centroids
- Assign items to closest centroid
- Repeat until clusters assignments are stable
Examples
km <- kmeans(t(scale(t(y))), 3)
km$cluster
## g1 g2 g3 g4 g5 g6 g7 g8 g9 g10 g11 g12 g13 g14 g15 g16 g17 g18 g19 g20
## 1 3 1 1 1 1 3 2 1 3 1 3 1 2 3 2 2 3 1 1
## g21 g22 g23 g24 g25 g26 g27 g28 g29 g30 g31 g32 g33 g34 g35 g36 g37 g38 g39 g40
## 1 2 1 2 2 3 2 1 1 3 3 3 2 3 1 1 3 3 1 1
## g41 g42 g43 g44 g45 g46 g47 g48 g49 g50 g51 g52 g53 g54 g55 g56 g57 g58 g59 g60
## 2 2 1 2 2 2 1 3 3 2 2 2 3 3 2 2 1 2 3 2
## g61 g62 g63 g64 g65 g66 g67 g68 g69 g70 g71 g72 g73 g74 g75 g76 g77 g78 g79 g80
## 2 3 3 3 3 3 2 1 1 2 1 3 1 1 1 2 3 3 1 2
## g81 g82 g83 g84 g85 g86 g87 g88 g89 g90 g91 g92 g93 g94 g95 g96 g97 g98 g99 g100
## 2 1 3 3 2 2 1 2 1 1 2 3 3 2 2 2 3 1 1 1
Fuzzy C-Means Clustering
- In contrast to strict (hard) clustering approaches, fuzzy (soft) clustering methods allow multiple cluster memberships of the clustered items (Hathaway, Bezdek, and Pal 1996).
- This is commonly achieved by assigning to each item a weight of belonging to each cluster.
- Thus, items at the edge of a cluster, may be in a cluster to a lesser degree than items at the center of a cluster.
- Typically, each item has as many coefficients (weights) as there are clusters that sum up for each item to one.
Examples
Fuzzy Clustering with fanny
library(cluster) # Loads the cluster library.
fannyy <- fanny(y, k=4, metric = "euclidean", memb.exp = 1.2)
round(fannyy$membership, 2)[1:4,]
## [,1] [,2] [,3] [,4]
## g1 0.97 0.02 0.02 0.00
## g2 0.07 0.91 0.01 0.00
## g3 0.28 0.08 0.44 0.20
## g4 0.84 0.03 0.12 0.01
fannyy$clustering # Hard clustering result
## g1 g2 g3 g4 g5 g6 g7 g8 g9 g10 g11 g12 g13 g14 g15 g16 g17 g18 g19 g20
## 1 2 3 1 3 3 1 2 1 2 3 4 1 2 4 2 2 4 1 4
## g21 g22 g23 g24 g25 g26 g27 g28 g29 g30 g31 g32 g33 g34 g35 g36 g37 g38 g39 g40
## 1 4 3 2 2 2 2 1 3 4 1 1 4 4 1 3 1 1 4 1
## g41 g42 g43 g44 g45 g46 g47 g48 g49 g50 g51 g52 g53 g54 g55 g56 g57 g58 g59 g60
## 2 4 1 2 2 3 3 1 2 2 3 4 4 1 4 3 3 4 2 2
## g61 g62 g63 g64 g65 g66 g67 g68 g69 g70 g71 g72 g73 g74 g75 g76 g77 g78 g79 g80
## 3 2 3 2 4 2 2 3 1 2 3 1 3 1 1 3 4 1 3 3
## g81 g82 g83 g84 g85 g86 g87 g88 g89 g90 g91 g92 g93 g94 g95 g96 g97 g98 g99 g100
## 3 1 2 2 2 2 3 2 1 3 4 2 2 2 2 2 2 1 3 1
(fannyyMA <- fannyy$membership > 0.20)[1:4,] # Soft clustering result
## [,1] [,2] [,3] [,4]
## g1 TRUE FALSE FALSE FALSE
## g2 FALSE TRUE FALSE FALSE
## g3 TRUE FALSE TRUE FALSE
## g4 TRUE FALSE FALSE FALSE
apply(fannyyMA, 1, which)[1:4] # First 4 clusters
## $g1
## [1] 1
##
## $g2
## [1] 2
##
## $g3
## [1] 1 3
##
## $g4
## [1] 1
Principal Component Analysis (PCA)
Principal components analysis (PCA) is a data reduction technique that allows to simplify multidimensional data sets to 2 or 3 dimensions for plotting purposes and visual variance analysis.
Basic Steps
- Center (and standardize) data
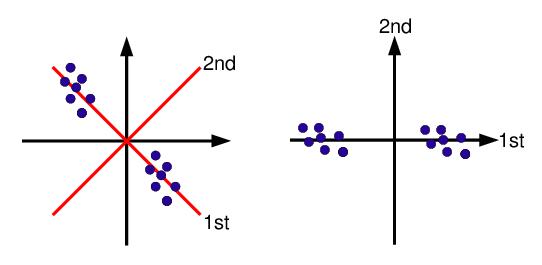
- First principal component axis
- Across centroid of data cloud
- Distance of each point to that line is minimized, so that it crosses the maximum variation of the data cloud
- Second principal component axis
- Orthogonal to first principal component
- Along maximum variation in the data
- First PCA axis becomes x-axis and second PCA axis y-axis
- Continue process until the necessary number of principal components is obtained
Example
pca <- prcomp(y, scale=T)
summary(pca) # Prints variance summary for all principal components
## Importance of components:
## PC1 PC2 PC3 PC4 PC5
## Standard deviation 1.179 1.1177 0.9965 0.8492 0.8041
## Proportion of Variance 0.278 0.2498 0.1986 0.1442 0.1293
## Cumulative Proportion 0.278 0.5279 0.7265 0.8707 1.0000
plot(pca$x, pch=20, col="blue", type="n") # To plot dots, drop type="n"
text(pca$x, rownames(pca$x), cex=0.8)
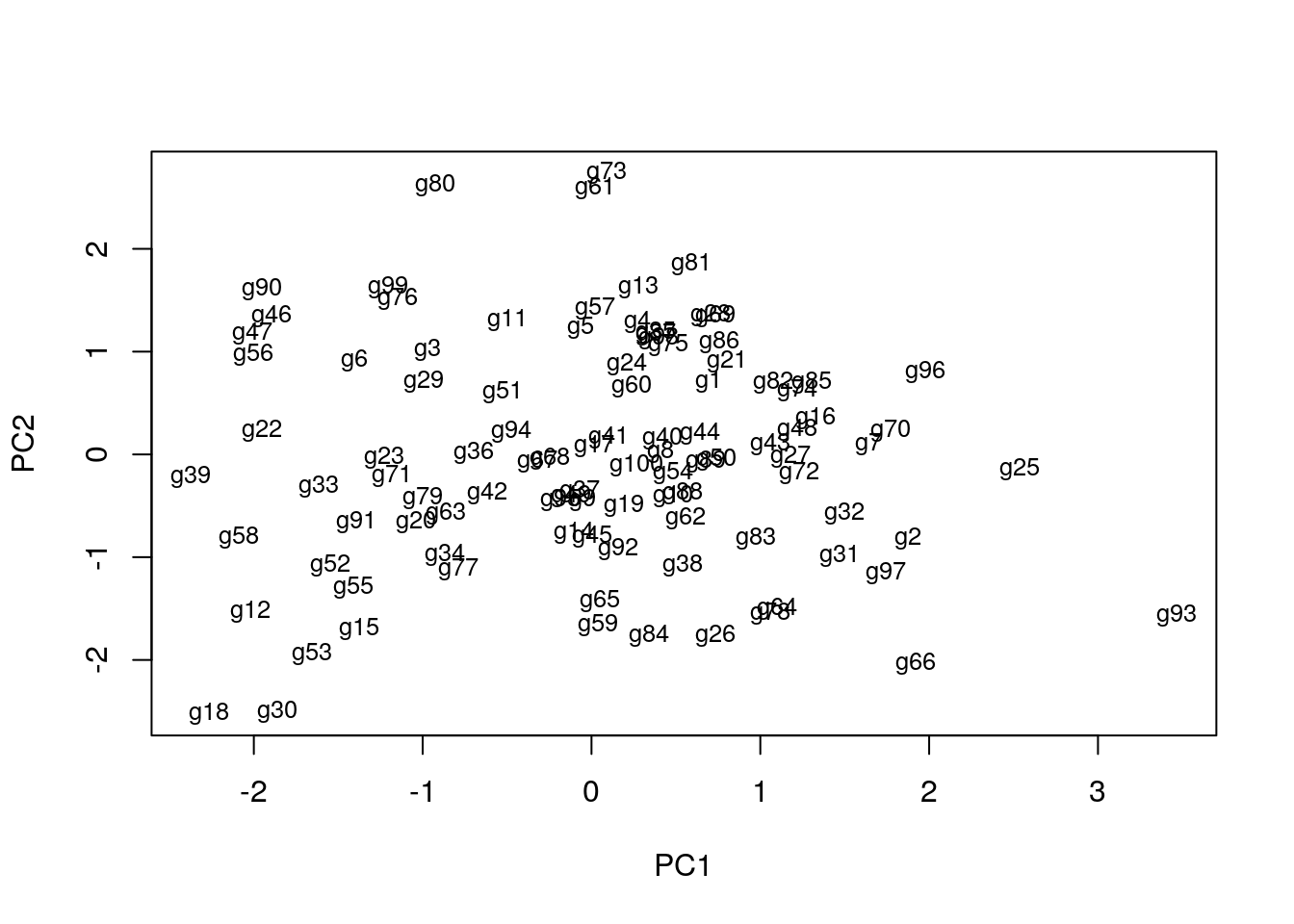 1st and 2nd principal components explain x% of variance in data.
1st and 2nd principal components explain x% of variance in data.
Multidimensional Scaling (MDS)
- Alternative dimensionality reduction approach
- Represents distances in 2D or 3D space
- Starts from distance matrix (PCA uses data points)
Example
The following example performs MDS analysis with cmdscale on the geographic distances among European cities.
loc <- cmdscale(eurodist)
plot(loc[,1], -loc[,2], type="n", xlab="", ylab="", main="cmdscale(eurodist)")
text(loc[,1], -loc[,2], rownames(loc), cex=0.8)

Biclustering
Finds in matrix subgroups of rows and columns which are as similar as possible to each other and as different as possible to the remaining data points.
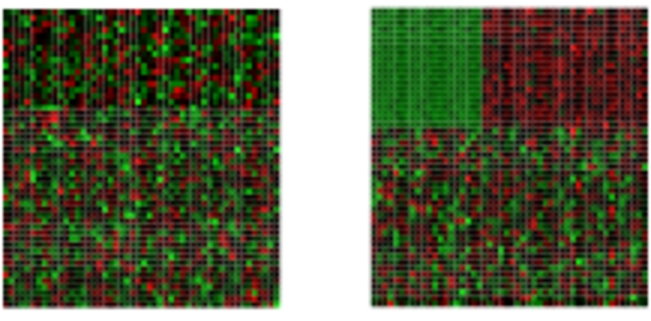
Unclustered ————————–> Clustered
Similarity Measures for Clusters
- Compare the numbers of identical and unique item pairs appearing in cluster sets
- Achieved by counting the number of item pairs found in both clustering sets (a) as well as the pairs appearing only in the first (b) or the second (c) set.
- With this a similarity coefficient, such as the Jaccard index, can be computed. The latter is defined as the size of the intersect divided by the size of the union of two sample sets: a/(a+b+c).
- In case of partitioning results, the Jaccard Index measures how frequently pairs of items are joined together in two clustering data sets and how often pairs are observed only in one set.
- Related coefficient are the Rand Index and the Adjusted Rand Index. These indices also consider the number of pairs (d) that are not joined together in any of the clusters in both sets.
Example:
Jaccard index for cluster sets
The following imports the cindex() function and computes the Jaccard Index for two sample cluster results.
source("http://faculty.ucr.edu/~tgirke/Documents/R_BioCond/My_R_Scripts/clusterIndex.R")
library(cluster); y <- matrix(rnorm(5000), 1000, 5, dimnames=list(paste("g", 1:1000, sep=""), paste("t", 1:5, sep=""))); clarax <- clara(y, 49); clV1 <- clarax$clustering; clarax <- clara(y, 50); clV2 <- clarax$clustering
ci <- cindex(clV1=clV1, clV2=clV2, self=FALSE, minSZ=1, method="jaccard")
ci[2:3] # Returns Jaccard index and variables used to compute it
## $variables
## a b c
## 6905 7441 7145
##
## $Jaccard_Index
## [1] 0.3212973
Clustering cluster sets with Jaccard index
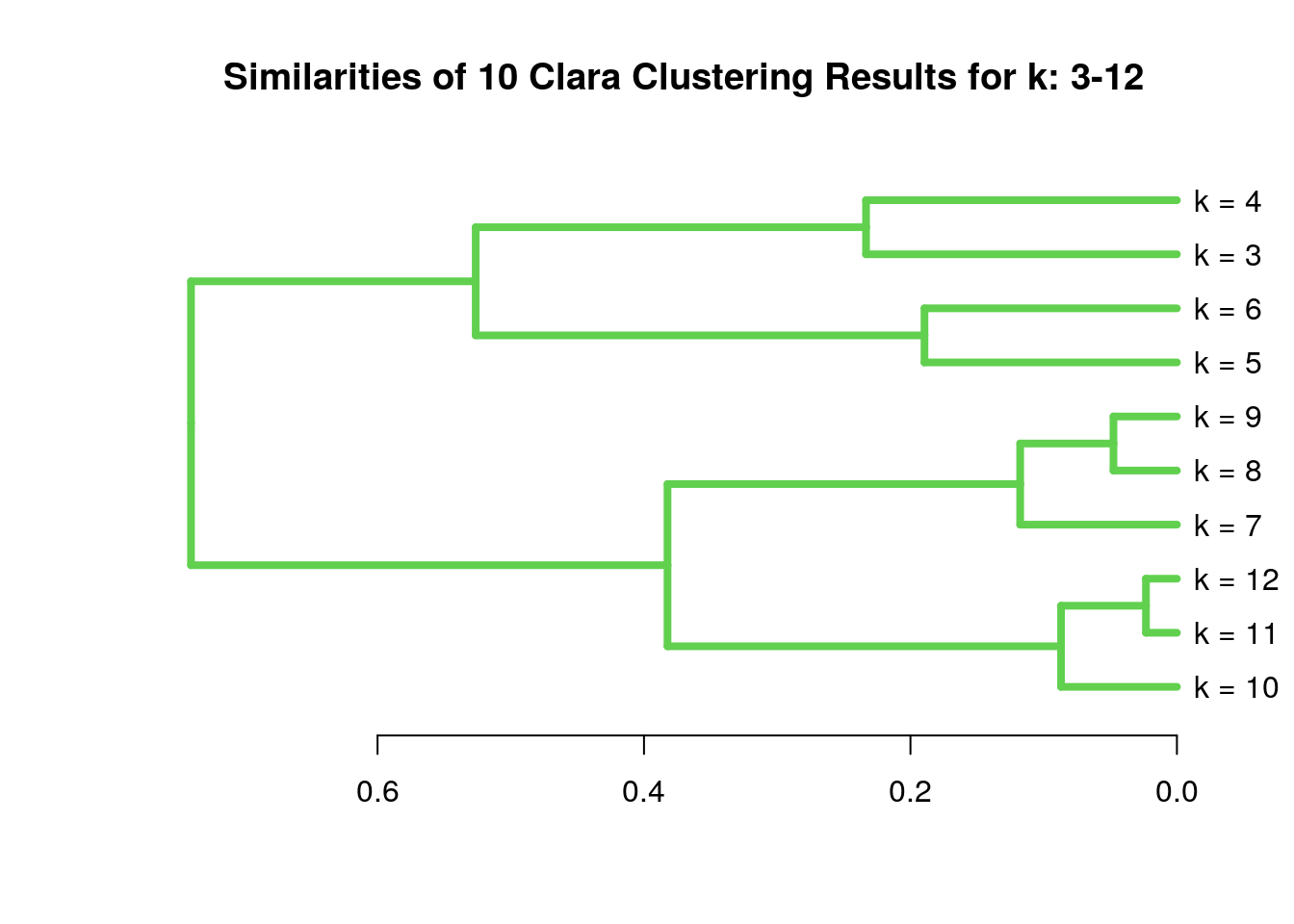
The following example shows how one can cluster entire cluster result sets. First, 10 sample cluster results are created with Clara using k-values from 3 to 12. The results are stored as named clustering vectors in a list object. Then a nested sapply loop is used to generate a similarity matrix of Jaccard Indices for the clustering results. After converting the result into a distance matrix, hierarchical clustering is performed with hclust.}
clVlist <- lapply(3:12, function(x) clara(y[1:30, ], k=x)$clustering); names(clVlist) <- paste("k", "=", 3:12)
d <- sapply(names(clVlist), function(x) sapply(names(clVlist), function(y) cindex(clV1=clVlist[[y]], clV2=clVlist[[x]], method="jaccard")[[3]]))
hv <- hclust(as.dist(1-d))
plot(as.dendrogram(hv), edgePar=list(col=3, lwd=4), horiz=T, main="Similarities of 10 Clara Clustering Results for k: 3-12")
- Remember: there are many additional clustering algorithms.
- Additional details can be found in the Clustering Section of the R/Bioconductor Manual.
Clustering Exercises
Data Preprocessing
Scaling
## Sample data set
set.seed(1410)
y <- matrix(rnorm(50), 10, 5, dimnames=list(paste("g", 1:10, sep=""),
paste("t", 1:5, sep="")))
dim(y)
## [1] 10 5
## Scaling
yscaled <- t(scale(t(y))) # Centers and scales y row-wise
apply(yscaled, 1, sd)
## g1 g2 g3 g4 g5 g6 g7 g8 g9 g10
## 1 1 1 1 1 1 1 1 1 1
Distance Matrices
Euclidean distance matrix
dist(y[1:4,], method = "euclidean")
## g1 g2 g3
## g2 4.793697
## g3 4.932658 6.354978
## g4 4.033789 4.788508 1.671968
Correlation-based distance matrix
Correlation matrix
c <- cor(t(y), method="pearson")
as.matrix(c)[1:4,1:4]
## g1 g2 g3 g4
## g1 1.00000000 -0.2965885 -0.00206139 -0.4042011
## g2 -0.29658847 1.0000000 -0.91661118 -0.4512912
## g3 -0.00206139 -0.9166112 1.00000000 0.7435892
## g4 -0.40420112 -0.4512912 0.74358925 1.0000000
Correlation-based distance matrix
d <- as.dist(1-c)
as.matrix(d)[1:4,1:4]
## g1 g2 g3 g4
## g1 0.000000 1.296588 1.0020614 1.4042011
## g2 1.296588 0.000000 1.9166112 1.4512912
## g3 1.002061 1.916611 0.0000000 0.2564108
## g4 1.404201 1.451291 0.2564108 0.0000000
Hierarchical Clustering with hclust
Hierarchical clustering with complete linkage and basic tree plotting
hr <- hclust(d, method = "complete", members=NULL)
names(hr)
## [1] "merge" "height" "order" "labels" "method" "call"
## [7] "dist.method"
par(mfrow = c(1, 2)); plot(hr, hang = 0.1); plot(hr, hang = -1)
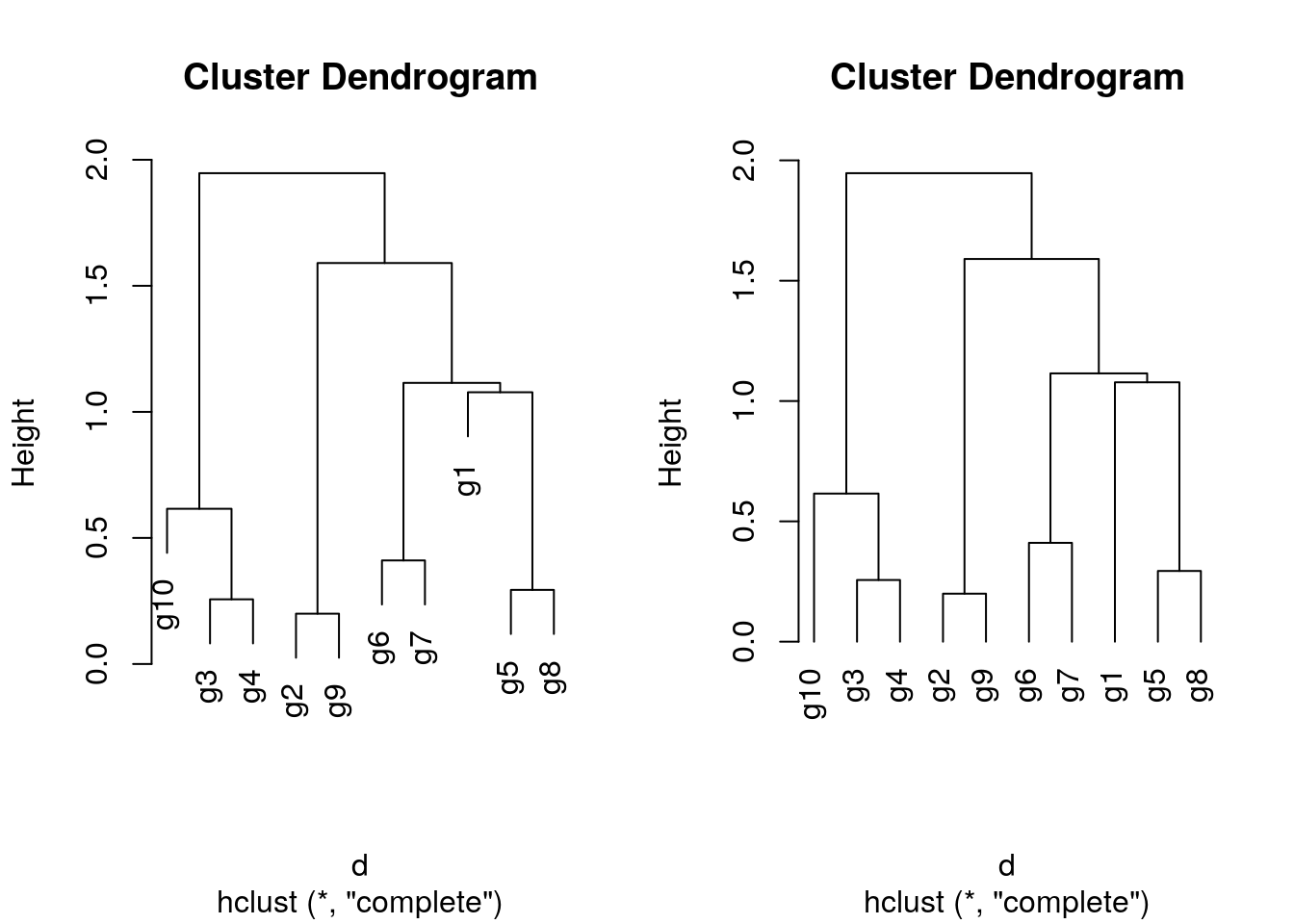
Tree plotting I
plot(as.dendrogram(hr), edgePar=list(col=3, lwd=4), horiz=T)
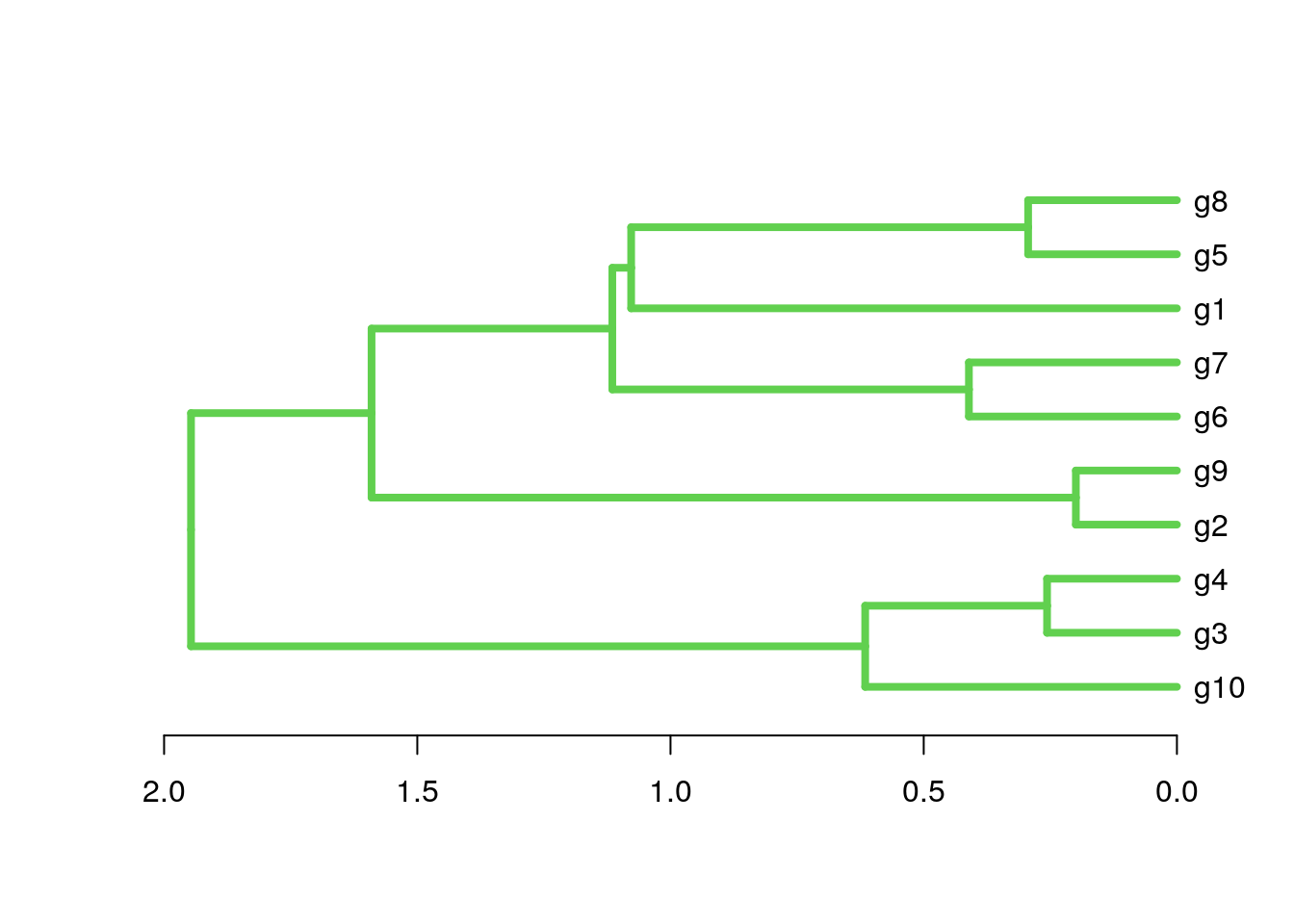
Tree plotting II
The ape library provides more advanced features for tree plotting
library(ape)
plot.phylo(as.phylo(hr), type="p", edge.col=4, edge.width=2,
show.node.label=TRUE, no.margin=TRUE)
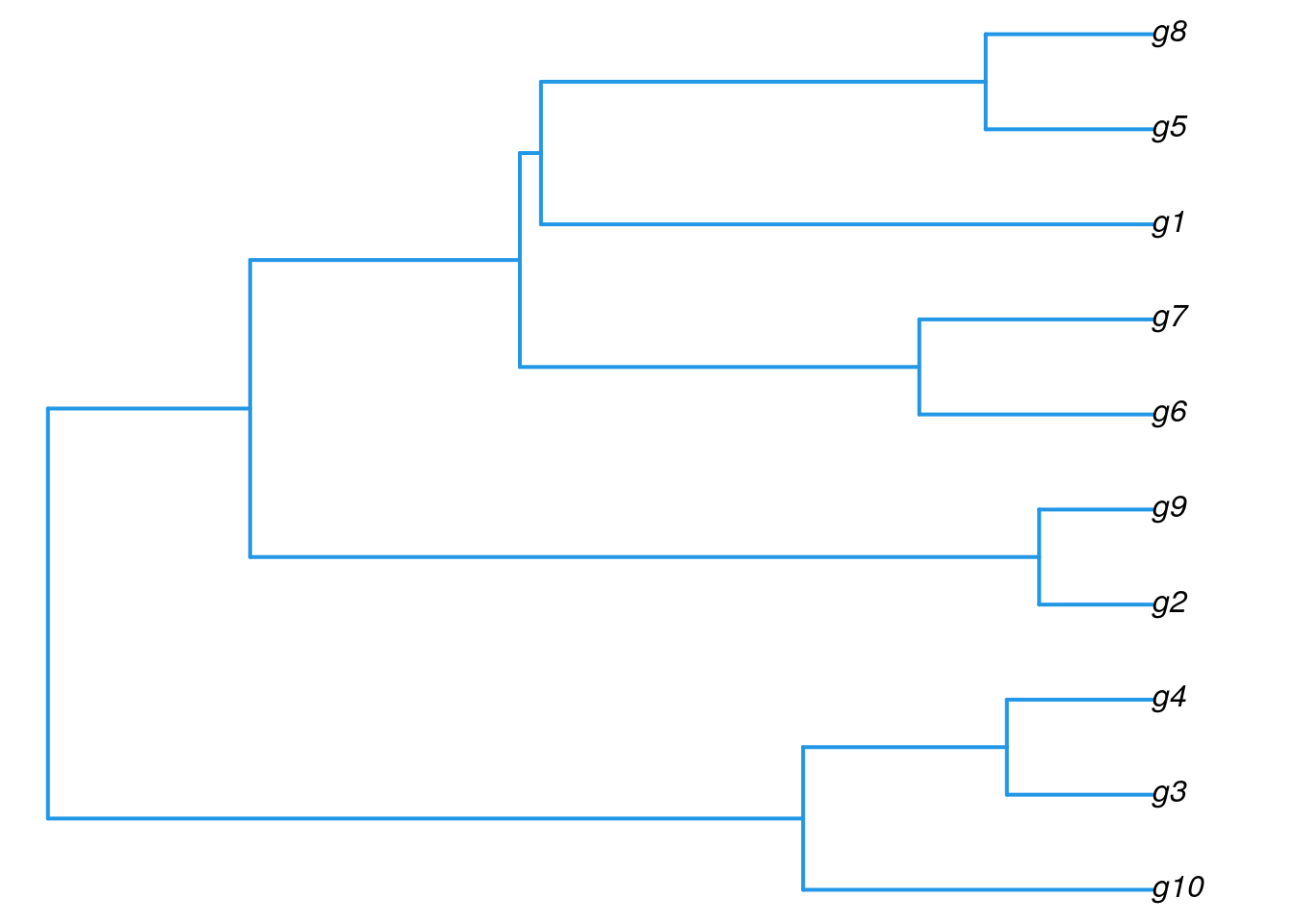
Tree Cutting
Accessing information in hclust objects
hr
##
## Call:
## hclust(d = d, method = "complete", members = NULL)
##
## Cluster method : complete
## Number of objects: 10
## Print row labels in the order they appear in the tree
hr$labels[hr$order]
## [1] "g10" "g3" "g4" "g2" "g9" "g6" "g7" "g1" "g5" "g8"
Tree cutting with cutree
mycl <- cutree(hr, h=max(hr$height)/2)
mycl[hr$labels[hr$order]]
## g10 g3 g4 g2 g9 g6 g7 g1 g5 g8
## 3 3 3 2 2 5 5 1 4 4
Heatmaps
With heatmap.2
All in one step: clustering and heatmap plotting
library(gplots)
heatmap.2(y, col=redgreen(75))
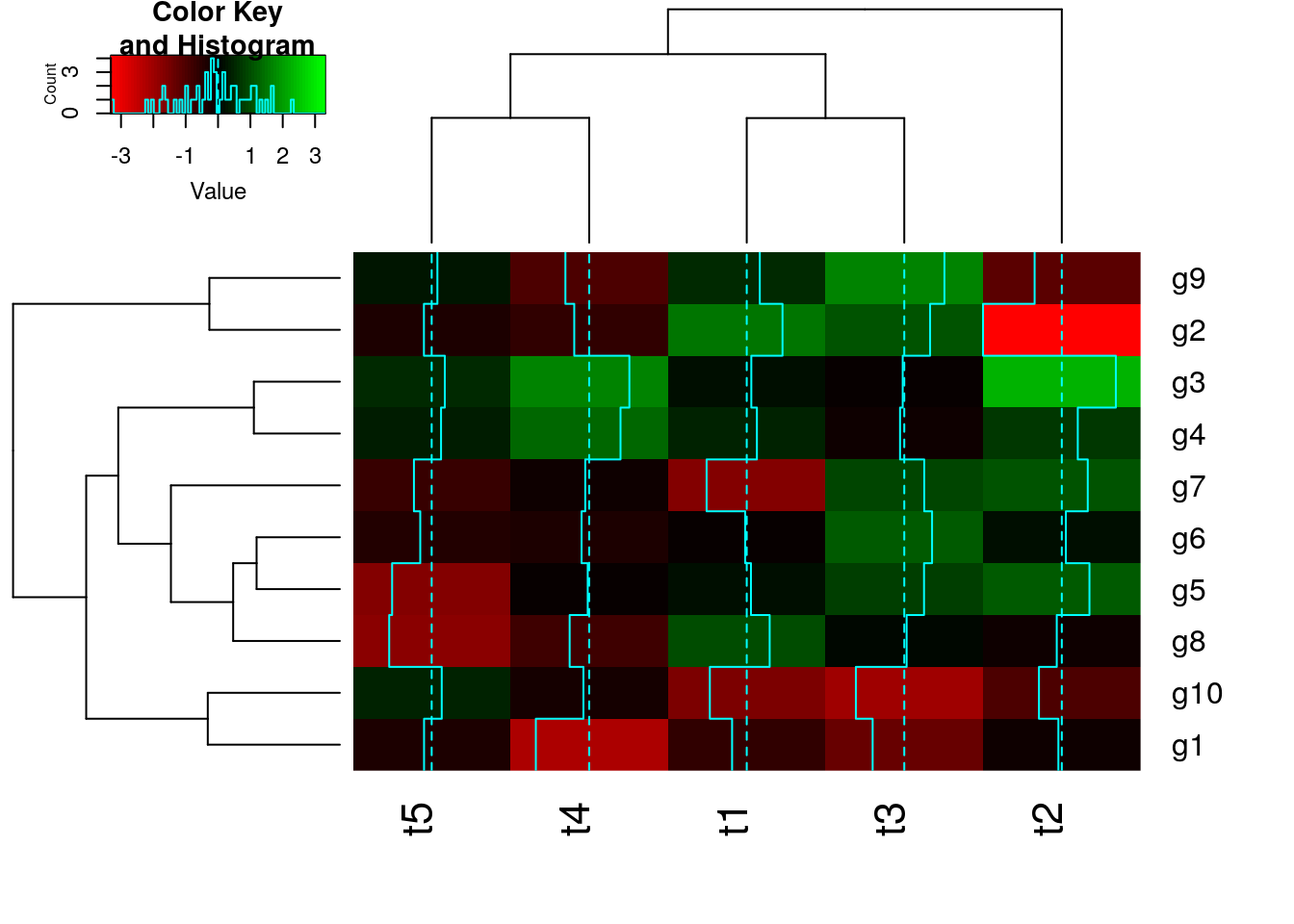
With pheatmap
All in one step: clustering and heatmap plotting
library(pheatmap); library("RColorBrewer")
pheatmap(y, color=brewer.pal(9,"Blues"))
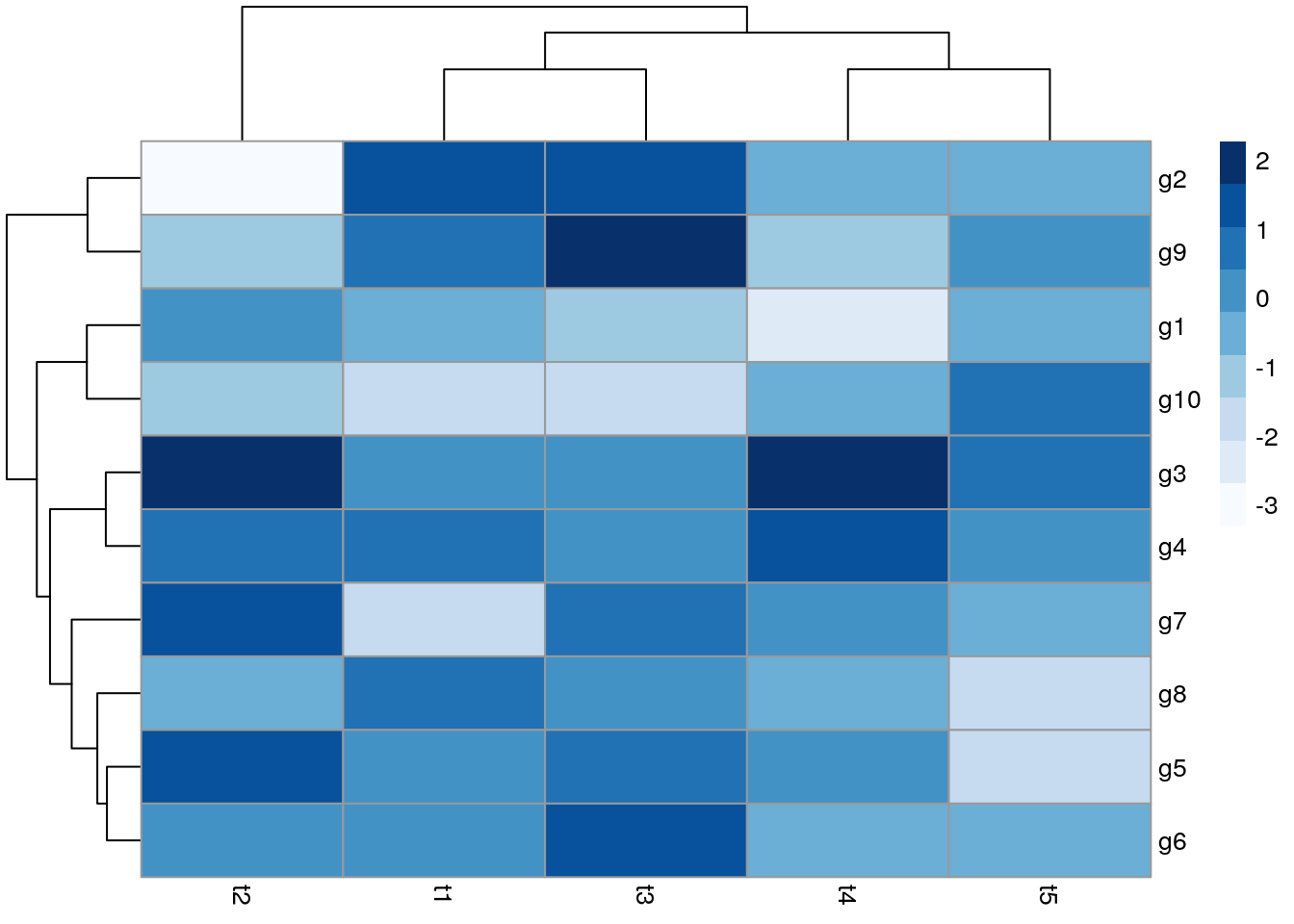
Customizing heatmaps
Customizes row and column clustering and shows tree cutting result in row color bar. Additional color schemes can be found here.
hc <- hclust(as.dist(1-cor(y, method="spearman")), method="complete")
mycol <- colorpanel(40, "darkblue", "yellow", "white")
heatmap.2(y, Rowv=as.dendrogram(hr), Colv=as.dendrogram(hc), col=mycol,
scale="row", density.info="none", trace="none",
RowSideColors=as.character(mycl))
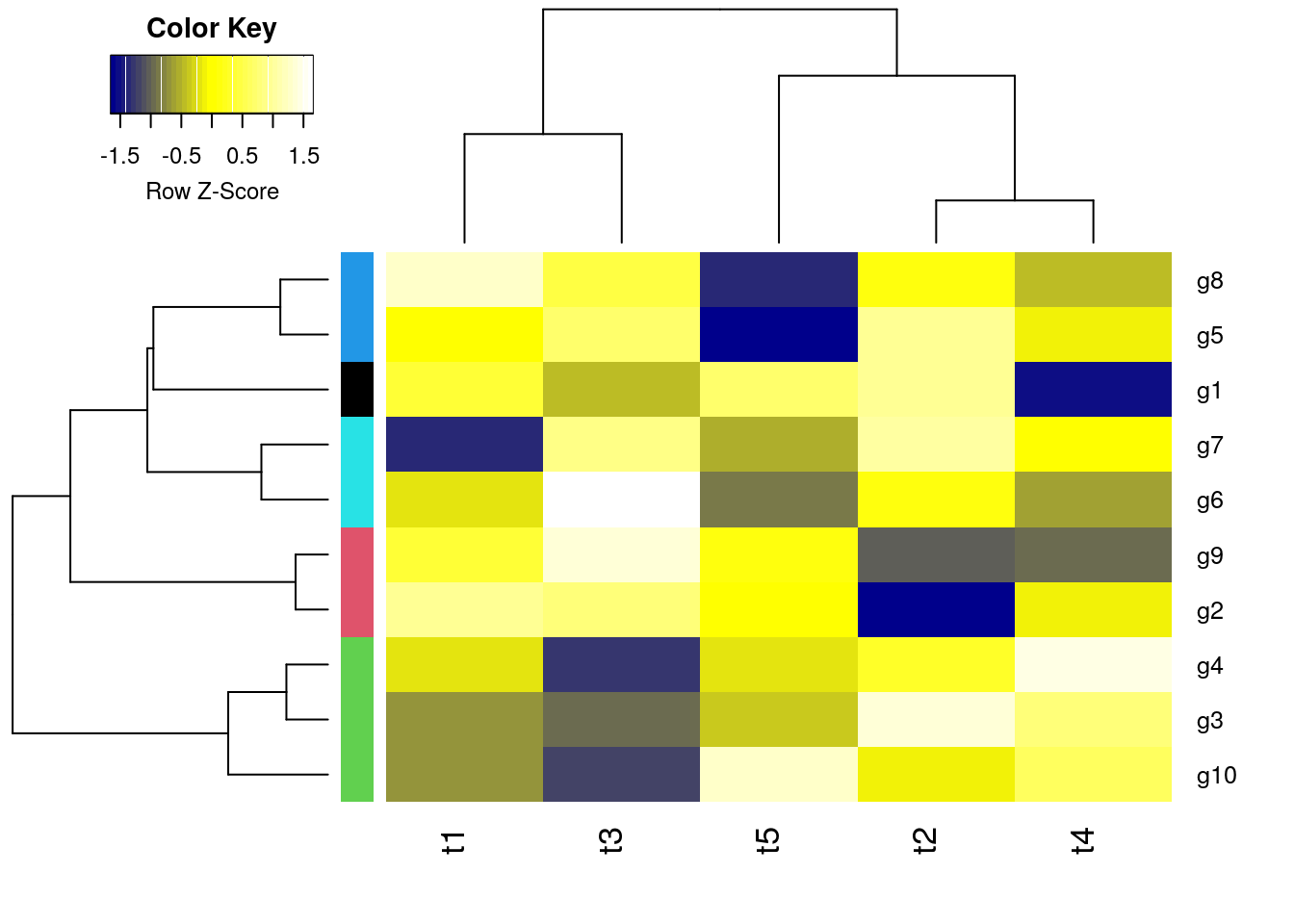
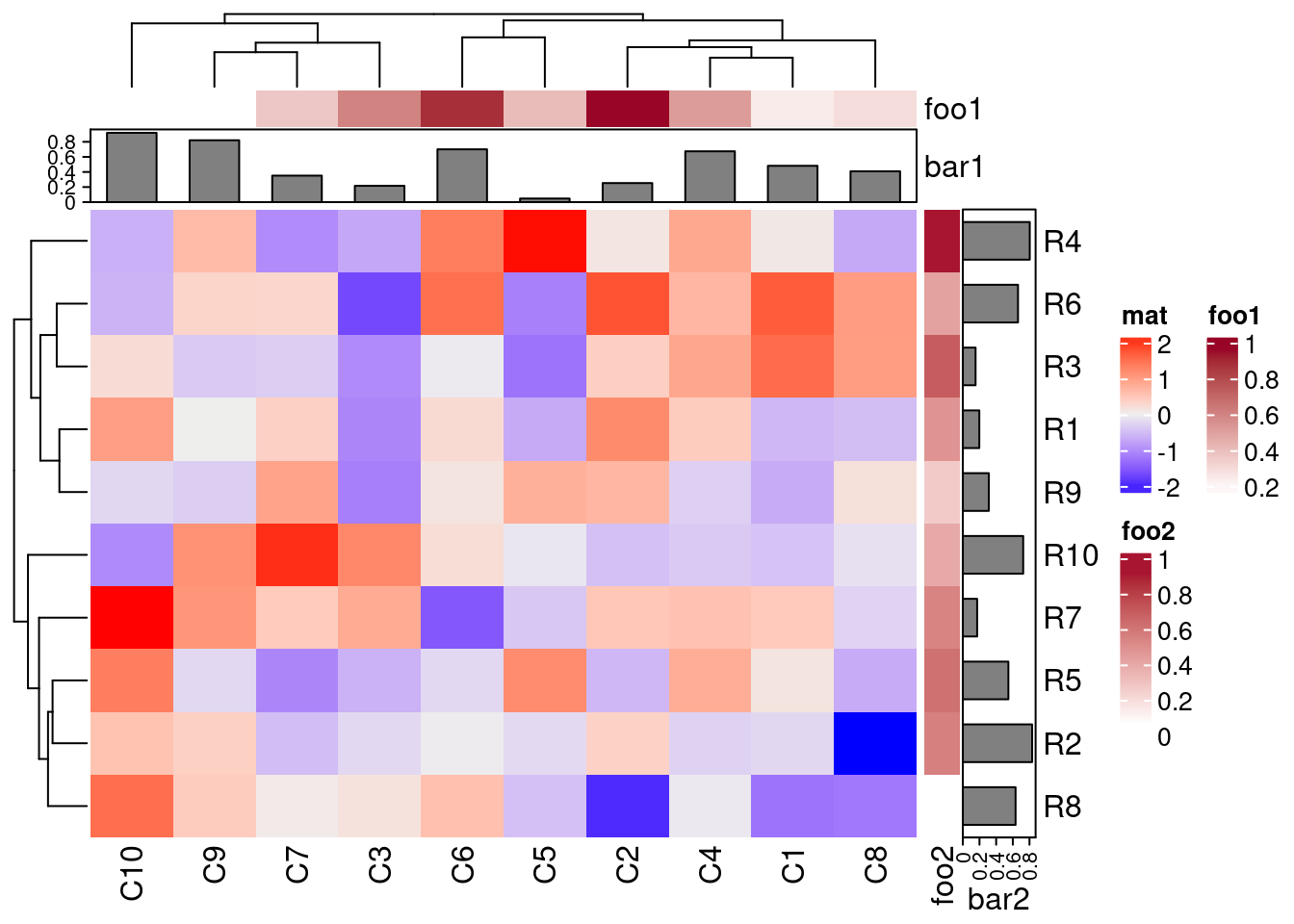
Complex heatmaps
For plotting complex heatmaps, the ComplexHeatmap provides useful functionalities. The following creates a sample heatmap. For additional examples, visit the manual here.
library(ComplexHeatmap)
set.seed(123)
mat = matrix(rnorm(100), 10)
rownames(mat) = paste0("R", 1:10)
colnames(mat) = paste0("C", 1:10)
column_ha = HeatmapAnnotation(foo1 = runif(10), bar1 = anno_barplot(runif(10)))
row_ha = rowAnnotation(foo2 = runif(10), bar2 = anno_barplot(runif(10)))
Heatmap(mat, name = "mat", top_annotation = column_ha, right_annotation = row_ha)
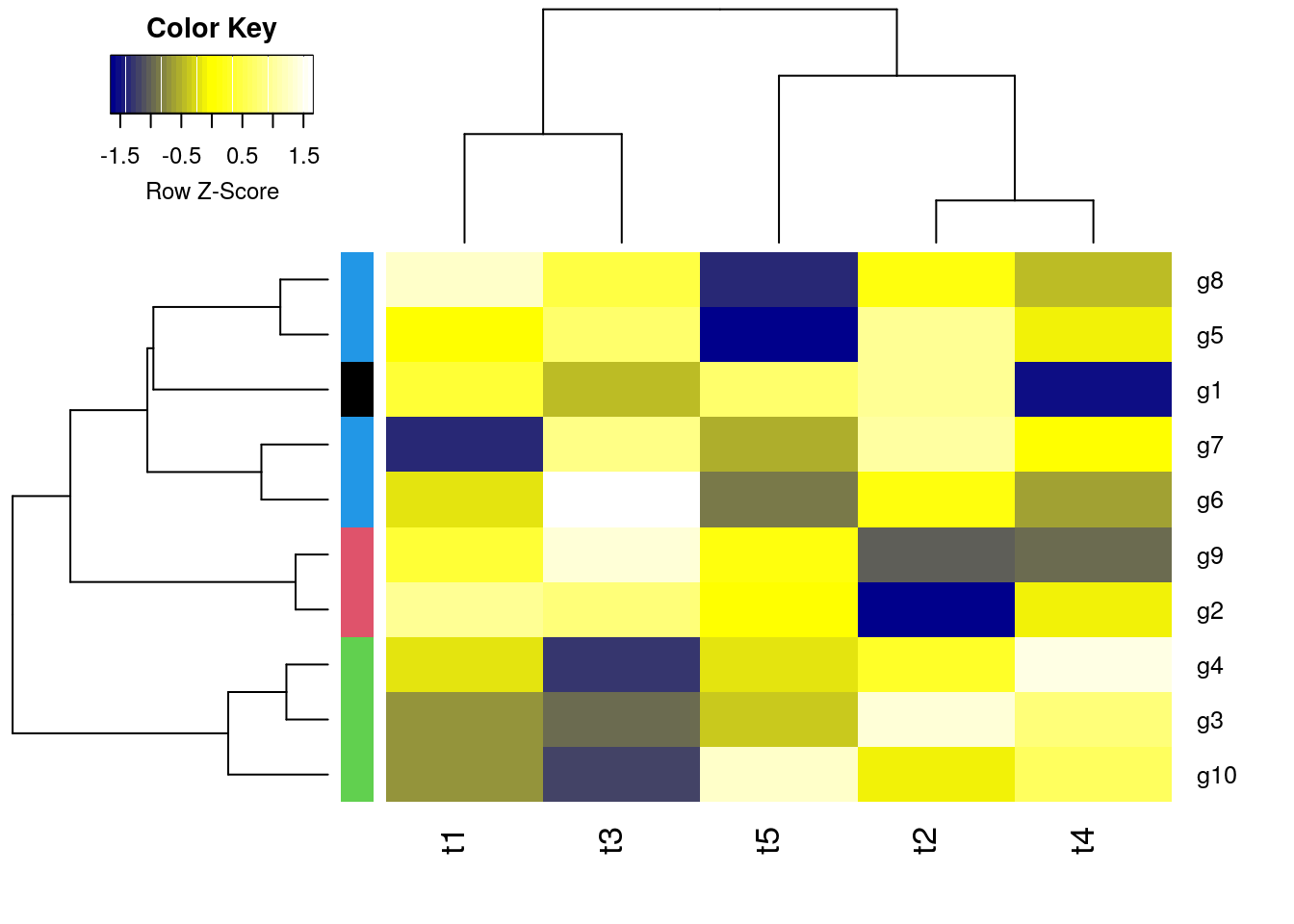
K-Means Clustering with PAM
Runs K-means clustering with PAM (partitioning around medoids) algorithm and shows result in color bar of hierarchical clustering result from before.
library(cluster)
pamy <- pam(d, 4)
(kmcol <- pamy$clustering)
## g1 g2 g3 g4 g5 g6 g7 g8 g9 g10
## 1 2 3 3 4 4 4 4 2 3
heatmap.2(y, Rowv=as.dendrogram(hr), Colv=as.dendrogram(hc), col=mycol,
scale="row", density.info="none", trace="none",
RowSideColors=as.character(kmcol))
K-Means Fuzzy Clustering
Performs k-means fuzzy clustering
library(cluster)
fannyy <- fanny(d, k=4, memb.exp = 1.5)
round(fannyy$membership, 2)[1:4,]
## [,1] [,2] [,3] [,4]
## g1 1.00 0.00 0.00 0.00
## g2 0.00 0.99 0.00 0.00
## g3 0.02 0.01 0.95 0.03
## g4 0.00 0.00 0.99 0.01
fannyy$clustering
## g1 g2 g3 g4 g5 g6 g7 g8 g9 g10
## 1 2 3 3 4 4 4 4 2 3
## Returns multiple cluster memberships for coefficient above a certain
## value (here >0.1)
fannyyMA <- round(fannyy$membership, 2) > 0.10
apply(fannyyMA, 1, function(x) paste(which(x), collapse="_"))
## g1 g2 g3 g4 g5 g6 g7 g8 g9 g10
## "1" "2" "3" "3" "4" "4" "4" "2_4" "2" "3"
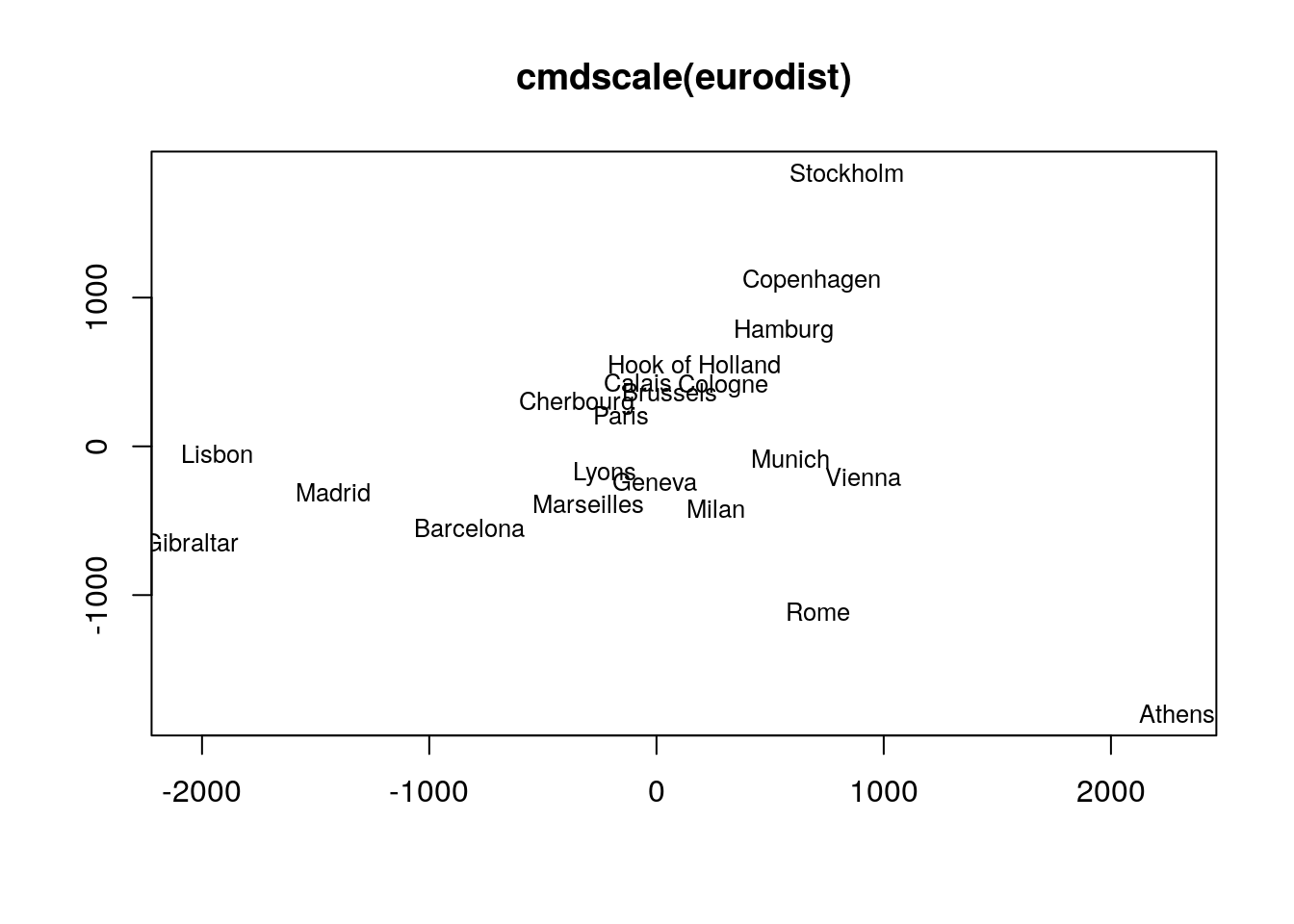
Multidimensional Scaling (MDS)
Performs MDS analysis on the geographic distances between European cities
loc <- cmdscale(eurodist)
## Plots the MDS results in 2D plot. The minus is required in this example to
## flip the plotting orientation.
plot(loc[,1], -loc[,2], type="n", xlab="", ylab="", main="cmdscale(eurodist)")
text(loc[,1], -loc[,2], rownames(loc), cex=0.8)
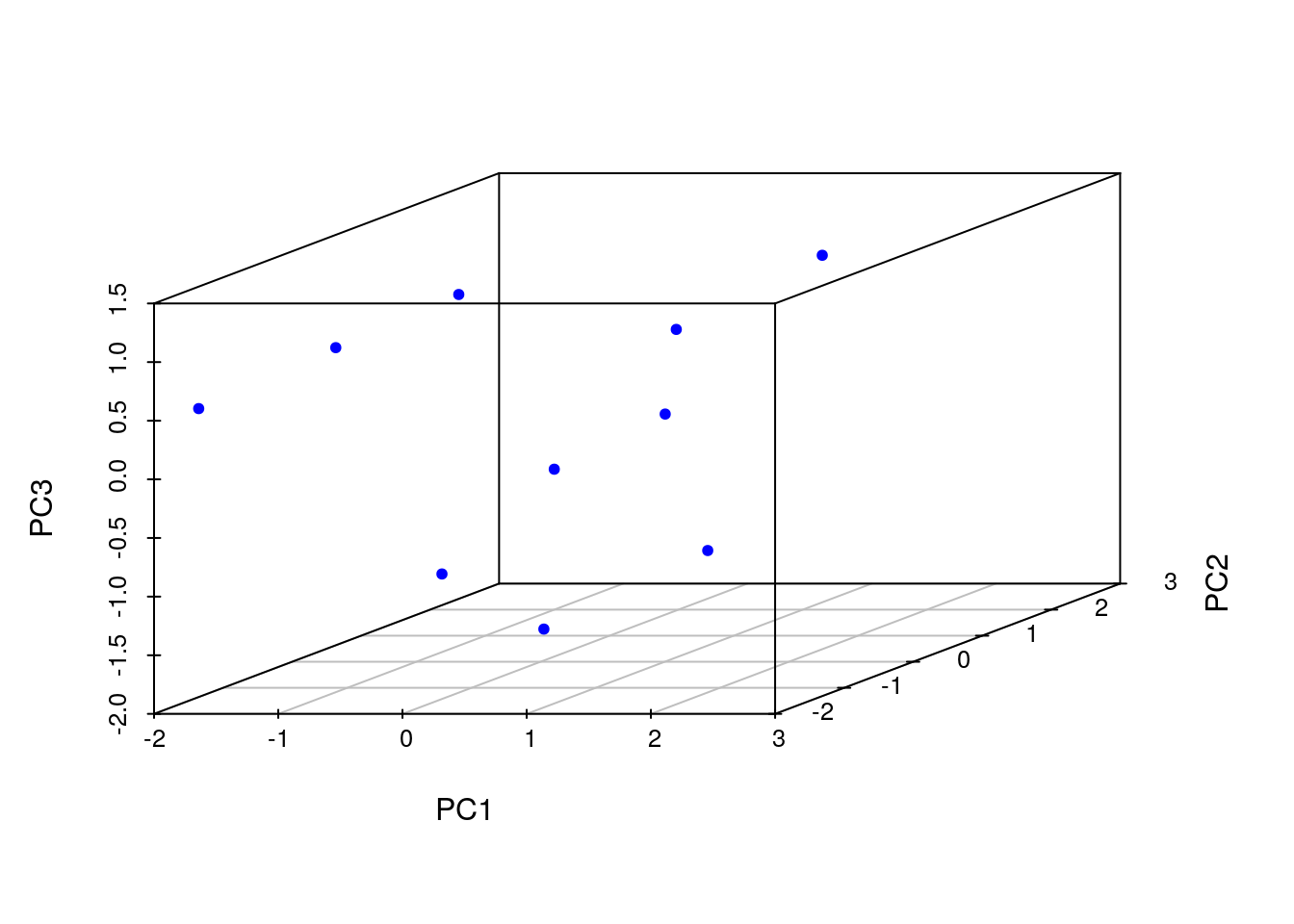
Principal Component Analysis (PCA)
Performs PCA analysis after scaling the data. It returns a list with class prcomp that contains five components: (1) the standard deviations (sdev) of the principal components, (2) the matrix of eigenvectors (rotation), (3) the principal component data (x), (4) the centering (center) and (5) scaling (scale) used.
library(scatterplot3d)
pca <- prcomp(y, scale=TRUE)
names(pca)
## [1] "sdev" "rotation" "center" "scale" "x"
summary(pca) # Prints variance summary for all principal components.
## Importance of components:
## PC1 PC2 PC3 PC4 PC5
## Standard deviation 1.3611 1.1777 1.0420 0.69264 0.4416
## Proportion of Variance 0.3705 0.2774 0.2172 0.09595 0.0390
## Cumulative Proportion 0.3705 0.6479 0.8650 0.96100 1.0000
scatterplot3d(pca$x[,1:3], pch=20, color="blue")
Additional Exercises
See here
Version Information
sessionInfo()
## R version 4.1.3 (2022-03-10)
## Platform: x86_64-pc-linux-gnu (64-bit)
## Running under: Debian GNU/Linux 10 (buster)
##
## Matrix products: default
## BLAS: /usr/lib/x86_64-linux-gnu/blas/libblas.so.3.8.0
## LAPACK: /usr/lib/x86_64-linux-gnu/lapack/liblapack.so.3.8.0
##
## locale:
## [1] LC_CTYPE=en_US.UTF-8 LC_NUMERIC=C LC_TIME=en_US.UTF-8
## [4] LC_COLLATE=en_US.UTF-8 LC_MONETARY=en_US.UTF-8 LC_MESSAGES=en_US.UTF-8
## [7] LC_PAPER=en_US.UTF-8 LC_NAME=C LC_ADDRESS=C
## [10] LC_TELEPHONE=C LC_MEASUREMENT=en_US.UTF-8 LC_IDENTIFICATION=C
##
## attached base packages:
## [1] stats graphics grDevices utils datasets methods base
##
## other attached packages:
## [1] scatterplot3d_0.3-41 RColorBrewer_1.1-2 pheatmap_1.0.12 cluster_2.1.2
## [5] gplots_3.1.1 ape_5.5 ggplot2_3.3.5 BiocStyle_2.22.0
##
## loaded via a namespace (and not attached):
## [1] gtools_3.9.2 tidyselect_1.1.1 xfun_0.30 bslib_0.3.1
## [5] purrr_0.3.4 lattice_0.20-45 colorspace_2.0-2 vctrs_0.3.8
## [9] generics_0.1.1 htmltools_0.5.2 yaml_2.3.5 utf8_1.2.2
## [13] rlang_1.0.2 jquerylib_0.1.4 pillar_1.6.4 glue_1.6.2
## [17] withr_2.4.3 DBI_1.1.1 lifecycle_1.0.1 stringr_1.4.0
## [21] munsell_0.5.0 blogdown_1.8.2 gtable_0.3.0 caTools_1.18.2
## [25] codetools_0.2-18 evaluate_0.15 knitr_1.37 fastmap_1.1.0
## [29] parallel_4.1.3 fansi_0.5.0 highr_0.9 Rcpp_1.0.8.2
## [33] KernSmooth_2.23-20 scales_1.1.1 BiocManager_1.30.16 jsonlite_1.8.0
## [37] digest_0.6.29 stringi_1.7.6 bookdown_0.24 dplyr_1.0.7
## [41] grid_4.1.3 cli_3.1.0 tools_4.1.3 bitops_1.0-7
## [45] magrittr_2.0.2 sass_0.4.0 tibble_3.1.6 crayon_1.4.2
## [49] pkgconfig_2.0.3 ellipsis_0.3.2 assertthat_0.2.1 rmarkdown_2.13
## [53] R6_2.5.1 nlme_3.1-155 compiler_4.1.3
References
Hathaway, R J, J C Bezdek, and N R Pal. 1996. “Sequential Competitive Learning and the Fuzzy c-Means Clustering Algorithms.” Neural Netw. 9 (5): 787–96. http://www.hubmed.org/display.cgi?uids=12662563.