Retrieve compound structures
results <- getAllCompoundIds(conn)
sdfset <- getCompounds(conn, results, keepOrder=TRUE)
sdfset
## An instance of "SDFset" with 1309 molecules
plot(sdfset[1:4], print=FALSE)
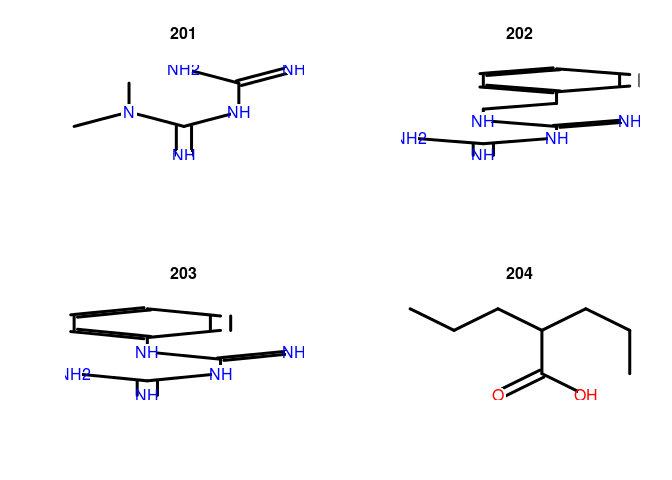
as.data.frame(datablock2ma(datablock(sdfset)))[1:4,]
## instance_id batch_id cmap_name INN1 concentration..M. duration..h. cell2 array3
## 201 1 1 metformin INN 1.00e-05 6 MCF7 HG-U133A
## 202 21 2 phenformin INN 1.00e-05 6 MCF7 HG-U133A
## 203 22 2 phenyl biguanide 1.00e-05 6 MCF7 HG-U133A
## 204 23 2 valproic acid INN 1.00e-03 6 MCF7 HG-U133A
## perturbation_scan_id vehicle_scan_id4 scanner vehicle vendor catalog_number
## 201 EC2003090503AA EC2003090502AA HP GeneArray Scanner medium Sigma-Aldrich D5035
## 202 EC2003091104AA EC2003091102AA HP GeneArray Scanner medium Sigma-Aldrich P7045
## 203 EC2003091105AA EC2003091102AA HP GeneArray Scanner medium Sigma-Aldrich P19906
## 204 EC2003091106AA EC2003091102AA HP GeneArray Scanner medium Sigma-Aldrich P4543
## catalog_name SOURCE_DRUG UNIPROT P_SCORE DIRECTIONALITY
## 201 1,1-dimethylbiguanide hydrochloride Metformin ZN396_HUMAN 1.53601286 Stimulatory
## 202 phenformin hydrochloride Phenformin KI2L3_HUMAN -6.33081681 Inhibitory
## 203 1-phenylbiguanide hydrochloride PHENYL BIGUANIDE RHOC_HUMAN -4.21084571 Inhibitory
## 204 2-propylpentanoic acid Valproic Acid GSK3B_HUMAN -58.47057966 Inhibitory
## PUBCHEM_ID DRUGBANK_ID DRUGBANK_GROUP ATCCODES chembank_id chembank_name
## 201 CID4091 DB00331 approved A10BD11|A10BA02 1714 metformin
## 202 CID8249 DB00914 approved|withdrawn A10BA01 1018627 phenformin
## 203 CID4780 32656 phenylbiguanide
## 204 CID3121 DB00313 approved|investigational N03AG01 471 valproic acid
## match_distance smiles
## 201 0 CN(C)C(=N)NC(=N)N
## 202 0 NC(=N)NC(=N)NCCc1ccccc1
## 203 0 NC(=N)NC(=N)Nc1ccccc1
## 204 0 CCCC(CCC)C(=O)O
Retrieve compound properties
myfeat <- listFeatures(conn)
feat <- getCompoundFeatures(conn, results, myfeat)
feat[1:4,]
## compound_id aromatic cansmi cansmins formula hba1 hba2 hbd
## 1 201 0 CN(C(=N)NC(=N)N)C CN(C(=N)NC(=N)N)C C4H11N5 5 5 4
## 2 202 1 N=C(NC(=N)N)NCCc1ccccc1 N=C(NC(=N)N)NCCc1ccccc1 C10H15N5 5 5 5
## 3 203 1 N=C(Nc1ccccc1)NC(=N)N N=C(Nc1ccccc1)NC(=N)N C8H11N5 5 5 5
## 4 204 0 CCCC(C(=O)O)CCC CCCC(C(=O)O)CCC C8H16O2 2 2 1
## inchi logp
## 1 InChI=1S/C4H11N5/c1-9(2)4(7)8-3(5)6/h1-2H3,(H5,5,6,7,8) 0.2565
## 2 InChI=1S/C10H15N5/c11-9(12)15-10(13)14-7-6-8-4-2-1-3-5-8/h1-5H,6-7H2,(H6,11,12,13,14,15) 1.9181
## 3 InChI=1S/C8H11N5/c9-7(10)13-8(11)12-6-4-2-1-3-5-6/h1-5H,(H6,9,10,11,12,13) 1.8800
## 4 InChI=1S/C8H16O2/c1-3-5-7(6-4-2)8(9)10/h7H,3-6H2,1-2H3,(H,9,10) 2.2874
## mr mw ncharges nf r2nh r3n rcch rcho rcn rcooh rcoor rcor rings rnh2 roh ropo3 ror
## 1 36.9285 129.1636 0 0 1 1 0 0 0 0 0 0 0 0 0 0 0
## 2 61.3212 205.2596 0 0 2 0 0 0 0 0 0 0 1 0 0 0 0
## 3 53.2452 177.2064 0 0 2 0 0 0 0 0 0 0 1 0 0 0 0
## 4 42.3418 144.2114 0 0 0 0 0 0 0 1 0 0 0 0 0 0 0
## title tpsa
## 1 metformin 88.99
## 2 phenformin 97.78
## 3 phenyl biguanide 97.78
## 4 valproic acid 37.30